Novel Kv1.3 inhibitor peptide with unique primary structure
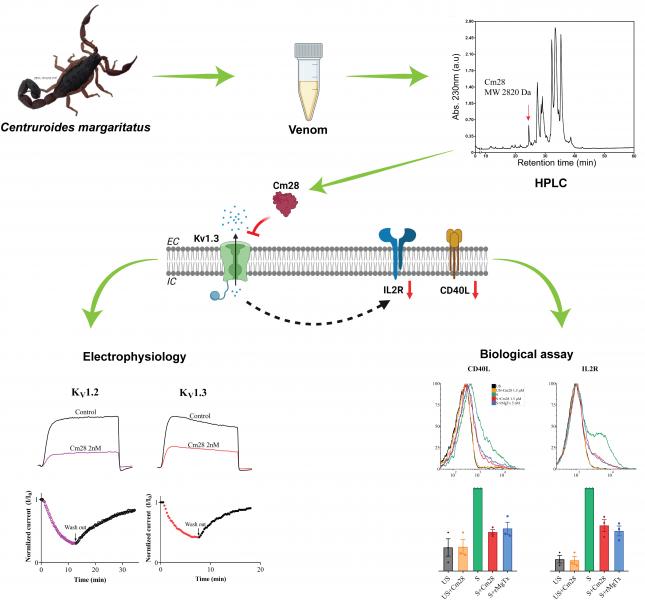
Muhammad Umair Naseem, Gyorgy Panyi and his colleagues studied a novel scorpion toxin peptide isolated from the venom of Centruroides margaritatus, Cm28, in international collaboration (Prof Possani’s team, UNAM, Mexico). The team is working on peptide toxins that inhibit the Kv1.3 potassium channels of lymphocytes, and thus, suppress the activation and proliferation of autoreactive T cells in autoimmune diseases. As opposed to the well-known K+ channel inhibitor peptides, Cm28 is shorter (27 amino acids only), has less than 40% similarity with other blockers and lacks the typical functional dyad (lysine-tyrosine) required to block KV channels. In spite of these, Cm28 blocks the pore of the voltage-gated K+ channels Kv1.2 and Kv1.3 with high affinity. In a biological functional assay Cm28 strongly downregulated the expression of the activation markers interleukin-2 receptor and CD40 ligand in activated human CD4+ effector memory T lymphocytes. Cm28, due to its unique structure, may serve as a template for the generation of novel peptides targeting Kv1.3 in autoimmune diseases. Their results were published in the Journal of General Physiology (DOI: https://doi.org/10.1085/jgp.202213146, https://doi.org/10.1085/jgp.202213220, reserach_newsjgp_202213220_pgy_umair.pdf).