Membrane dynamics Research Group

Head of the group
László Mátyus MD, PhD, DSc
E-mail:
Webpage: https://biophys.med.unideb.hu/en/membrane-dynamics-research-group

Staff
- Attila Jenei, MSc, PhD, full professor
- Andrea Dóczy-Bodnár, MSc, PhD, senior research fellow
- Enikő Nizsalóczki, MSc, PhD, assistant lecturer / educational manager
- István Csomós, MSc, junior research fellow
- József Kormos, PhD student
- Adrienn Bagosi, BSc, laboratory assistant
Research projects
- Biophysical and functional analysis of localization and molecular interactions of IL-9 receptor in human T cells
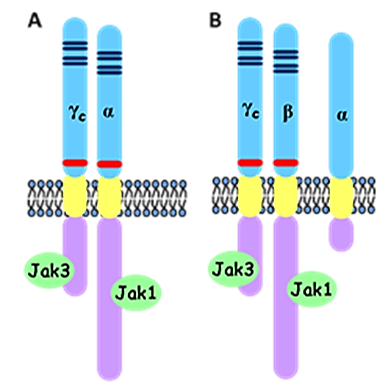 T cell function and homeostasis is regulated by a series of signals delivered by cytokines, a subset of which acts through receptor complexes sharing the common γ chain (γc). These include IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. Besides γc, IL-2 and -15 receptors share an additional signaling subunit (IL-2/15Rβ) and both have their own, “private” α-chains ensuring high affinity binding of the appropriate cytokine. In contrast, other members of the family, such as IL-9, exert their activities via heterodimeric receptor complexes consisting of a cytokine-specific α chain interacting with γc for signaling (Figure 1).
T cell function and homeostasis is regulated by a series of signals delivered by cytokines, a subset of which acts through receptor complexes sharing the common γ chain (γc). These include IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. Besides γc, IL-2 and -15 receptors share an additional signaling subunit (IL-2/15Rβ) and both have their own, “private” α-chains ensuring high affinity binding of the appropriate cytokine. In contrast, other members of the family, such as IL-9, exert their activities via heterodimeric receptor complexes consisting of a cytokine-specific α chain interacting with γc for signaling (Figure 1).
Using various approaches, we previously revealed significant similarities in the molecular environments of IL-2 and IL-15 receptors in human T lymphoma cells. We showed preassembly of the heterotrimeric IL-2R and IL-15R, the colocalization of their alpha chains occurring at different size scales, and the existence of supramolecular complexes of IL-2R, IL-15R, and MHC glycoproteins in lipid rafts of T cells. The question arises, whether we can find a similar behavior for the heterodimeric IL-9R, another member of the common gamma cytokine receptor family. Since the expression of IL-9Rα is mainly restricted to T cell subsets also expressing the heterotrimeric IL-2R, in which IL-2 has a crucial regulatory role, it is an intriguing question whether IL-9R and IL-2R function independently of each other or they combine to form a common complex similar to that suggested previously for the IL-2/15R system.
Our confocal microscopic co-localization and FRET experiments demonstrated interaction of IL-9Rα with IL-2R and MHC glycoproteins in lipid rafts of MT-2 and Kit225/IL-9R human T lymphoma cells suggesting that IL-9Rα is another component of the aforesaid IL-2R/MHC superclusters. Interestingly enough, IL-9Rα could also be detected in membrane areas segregated spatially from IL-2R/MHC-rich membrane regions (Figure 2). This bipartite nature of IL-9R distribution was reflected in the signaling capacity of the receptor complex as well: our STAT1 phosphorylation experiments indicated accommodation of IL-9Rα in separate membrane domains with different cholesterol-sensitivities (probably raft and non-raft regions).
Complementing Pearson correlation data with co-occurrence analysis of confocal microscopic images revealed that a minimum degree of IL-9R/IL-2R co-localization exists at the cell surface regardless of the overall spatial correlation of the two receptor kinds. Moreover, our FRET experiments demonstrated molecular scale assemblies of the elements of the IL-9/IL-2R system. Binding of IL-9 altered the structure and/or composition of these clusters. Further investigations are in progress to elucidate the functional consequences of the bipartite nature of IL-9Ralpha organization as well as the details and factors regulating the distribution between the different types of membrane areas.
- Targeting the IL-6R/STAT3 signaling pathway by benzophenanthridine alkaloids in uveal melanoma cells
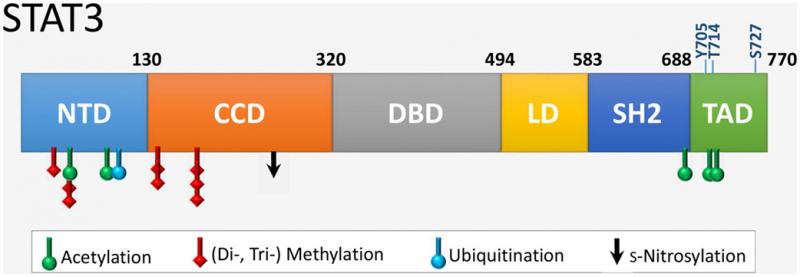
- STAT3 is a transcription factor with a critical role in the regulation of cell cycle, cell proliferation/survival and cell migration. As a consequence, it is a promising target in antitumor therapies. Canonical function of STAT3 demands phosphorylation of a Tyr705 residue followed by dimerization and nuclear translocation (Figure 3). STAT3 can also be phosphorylated on Ser727, with a putative role in fine tuning STAT3 activation, however its exact role is not clarified yet. One of the major activators of STAT3 is interleukin-6, a cytokine occurring in an elevated concentration in various tumors, including uveal melanomas. Chelidonine and sanguinarine, two of the benzophenantridine alkaloid components of Chelidonium majus, were reported previously to induce apoptosis and necrosis in various tumor cells, including uveal melanomas. Using flow (and image) cytometry methods we aim to study the interference of these alkaloids with the IL-6R/STA3 signaling pathway in uveal melanoma cell lines.
Representative publications
- Nizsalóczki E, Nagy P, Mocsár G, Szabó Á, Csomós I, Waldmann TA, Vámosi G, Mátyus L, Bodnár A. Minimum degree of overlap between IL-9R and IL-2R on human T lymphoma cells: a quantitative CLSM and FRET analysis. Cytometry A. 2018, 93 (11):1106-1117.
- Nizsalóczki E, Csomós I, Nagy P, Fazekas Z, Goldman CK, Waldmann TA, Damjanovich S, Vámosi G, Mátyus L, Bodnár A. Distinct Spatial Relationship of the Interleukin-9 Receptor with Interleukin-2 Receptor and Major Histocompatibility Complex Glycoproteins in Human T Lymphoma Cells. CHEMPHYSCHEM (doi: 10.1002/cphc.201402501) (2014)
- de Bakker BI, Bodnar A, van Dijk EMHP, Vamosi G, Damjanovich S, Waldmann TA, van Hulst NF, Jenei A, Garcia-Parajo MF. Nanometer-scale organization of the alpha subunits of the receptors for IL2 and IL15 in human T lymphoma cells. JOURNAL OF CELL SCIENCE 121: 627-633. (2008)
- Vámosi G*, Bodnár A*, Vereb G, Jenei A, Goldman CK, Langowski J, Tóth K, Mátyus L, Szöllősi J, Waldmann TA, Damjanovich S. IL-2 and IL-15 receptor alpha-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA 101: 11082-11087. (2004) (*contributed equally)
- Nagy P*, Mátyus L*, Jenei A*, Panyi G, Varga S, Matko J, Szollosi J, Gaspar R, Jovin T M, Damjanovich S. Cell fusion experiments reveal distinctly different association characteristics of cell-surface receptors. JOURNAL OF CELL SCIENCE 114: 4063-4071. (2001) (*contributed equally)
Recent publications
- Nizsalóczki E, Nagy P, Mocsár G, Szabó Á, Csomós I, Waldmann TA, Vámosi G, Mátyus L, Bodnár A. Minimum degree of overlap between IL-9R and IL-2R on human T lymphoma cells: a quantitative CLSM and FRET analysis. Cytometry A. 2018, 93 (11):1106-1117.
- Nagy É, Mocsár G, Sebestyén V, Volkó J, Papp F, Tóth K, Damjanovich S, Panyi G, Waldmann TA, Bodnár A, Vámosi G. Membrane Potential Distinctly Modulates Mobility and Signaling of IL-2 and IL-15 Receptors in T Cells. Biophys J. 2018, 114:2473-2482.
- Hrubi E, Imre L, Robaszkiewicz A, Virág L, Kerényi F, Nagy K, Varga G, Jenei A, Hegedüs C. Diverse effect of BMP-2 homodimer on mesenchymal progenitors of different origin. Hum Cell. 2018, 31:139-148.
- Nizsalóczki E, Csomós I, Nagy P, Fazekas Z, Goldman CK, Waldmann TA, Damjanovich S, Vámosi G, Mátyus L, Bodnár A. Distinct Spatial Relationship of the Interleukin-9 Receptor with Interleukin-2 Receptor and Major Histocompatibility Complex Glycoproteins in Human T Lymphoma Cells. CHEMPHYSCHEM 2014, 15: 3969-3978.
- Mocsár G, Volkó J, Rönnlund D, Widengren J, Nagy P, Szöllősi J, Tóth K, Goldman CK, Damjanovich S, Waldmann TA, Bodnár A, Vámosi G. MHC I Expression Regulates Co-clustering and Mobility of Interleukin-2 and -15 Receptors in T Cells. Biophys J. 2016, 111:100-12.
Updated: 2022.01.25.


